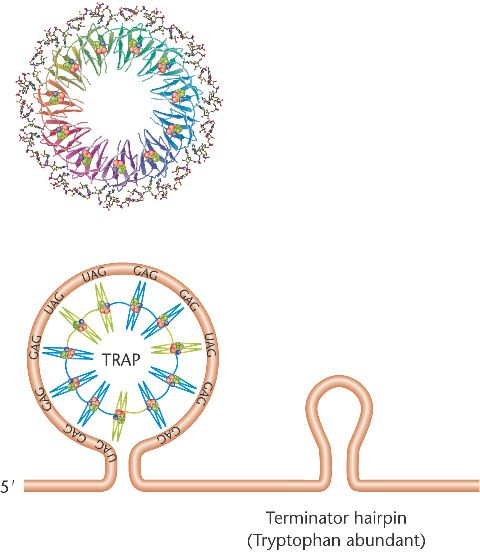
 The trp RNA-binding attenuation protein (TRAP) of B. subtilis
consists of 11 subunits, each capable of binding one molecule of tryptophan.
The trp RNA-binding attenuation protein (TRAP) of B. subtilis
consists of 11 subunits, each capable of binding one molecule of tryptophan.
saturated with tryptophan,
TRAP can bind to the 5'-leader sequence of the mRNA,
forming a terminator hairpin and lead to attenuation of expression of the trp operon.
If tryptophan is scarce,
an anti-TRAP (AT) protein inhibits binding of TRAP
to the leader sequence, stopping the attenuation.